Concentration calculations
Definition
You can also find most of this information on page 238 and 239 in your textbook.
You may be most used to the word concentration used in terms of paying attention to something. For example, you may concentrate on your study the night before a test. What are you doing when you concentrate? You are trying to focus all your attention on that one thing.
To concentrate can been seen as to focus all of your attention in a small area. Taking that definition into science, to concentrate is to put a lot of stuff (we call this the solute) into a smaller portion of the stuff you are mixing into (the solvent).
To calculate the concentration, simply divide the amount of solute by the total amount of the solution:
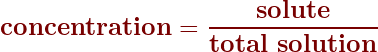
Concentrations as a percent
We frequently express concentrations as a percent. Percent means ‘per hundred.’ If you calculate your grade, say you got 40/50, into a percent, what you are really saying is ‘if the test were worth 100 points, I would have earned 80 points.’ The equation is, therefore:
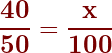
Then you ‘cross-multiply and divide to get the answer of 80%.
To calculate the concentration as a percent, the equation starts with:
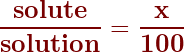
So, we generally rearrange this formula (by multiplying both sides by 100) and use the following to solve any solution percent concentration.
The equation for percent concentration, therefore, is
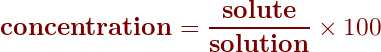
Calculating concentrations as percents
Video
The following video shows how to calculate mass percents. Don’t worry about the when she talks about PPM (stands for Parts Per Million), but the rest of the video is helpful.
Moles: Counting molecules, atoms, and ions
Chemicals are made up of such small particles that we cannot count them individually. Lets look at some examples to help you understand this huge counting number.
Dozen

Can you imagine going to the grocery store and asking to buy one egg? Eggs are small enough that they sell them in dozens (sometimes half-dozen, two-dozen, and two and one half-dozen).
Score

Score is a word that means 20. It’s not used much these days, but has an memorable role in US history, used by President Lincoln in the Gettysburg Address.
Gross

Small parts or materials sold to businesses are often sold by the gross. A gross is a dozen dozens. A jeweler might by a gross of gold studs, or a school might by a gross of pencils.
One other counting word based on the dozen is the great gross, which is a dozen gross.
The mole

You’ll learn much more about this in chemistry, but the mole is just a very large number. To get a sense of how large this number is, if you could count all the grains of sand on the earth, this would only be about 1 ten-thousandth of a mole. That means it would take 10,000 earths to have one mole of sand grains.
A twelve-ounce can of soda has about 20 moles of water! Hopefully that gives you an idea how small molecules, ions, and ions are.
Concentrations in moles/liter
One of the common measurements for concentration is the mole per liter. You can read about this on Page 239 in your textbook. To calculate the concentration in moles/liter, it’s a simple division calculation:
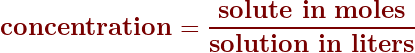
Video: Solving for molarity
The following video provides a good overview. At about 4:45 into the video, it gets into chemical formulas that you won’t need to do in this class (again, you’ll get to this in chemistry).
Online molarity simulation
If you would like to experiment with solutions, this PhET simulation is a great tool that allows you to adjust the amount of water, solutes, evaporation, and see the results with a meter.
