More coming soon!
Contents of this post
Definition of acids and bases
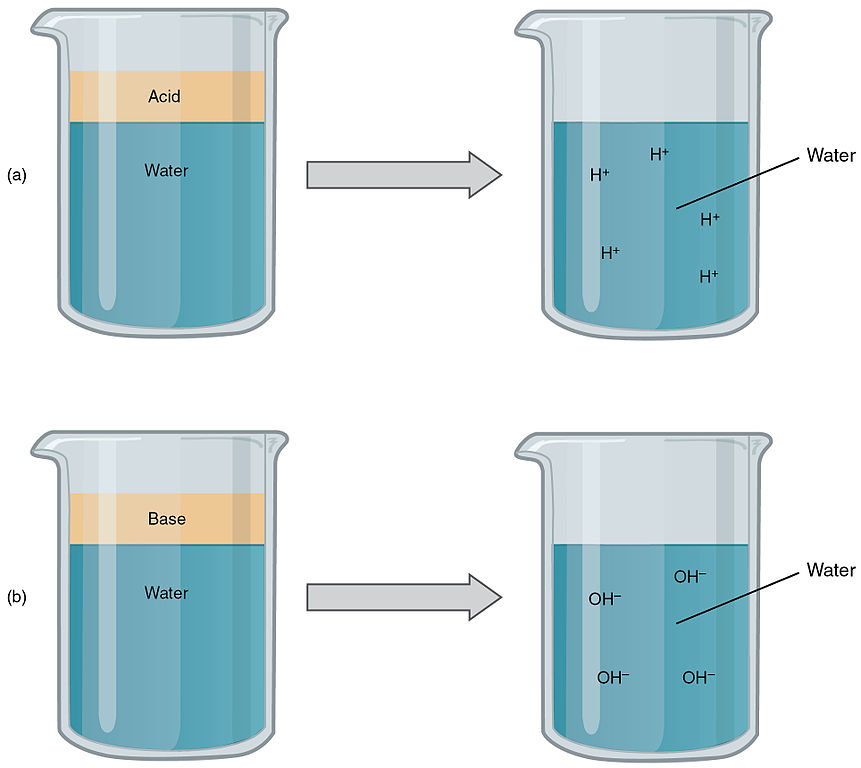
In the simplest definition, acids are substances that when dissolved in water release hydrogen ions (H+), and bases are substances that release hydroxide ions (OH-).
In chemistry class, you will dig deeper into acids and bases, and develop a more complete definition of acids and bases, but these two definitions will do for this class.
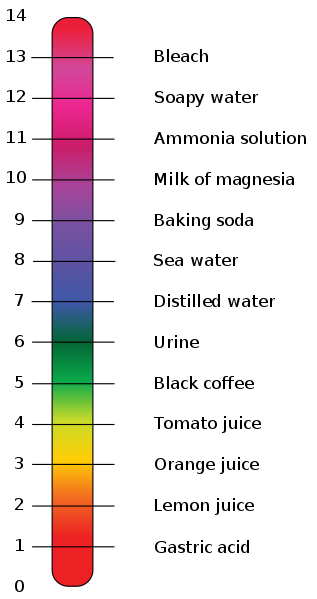
The pH scale was developed as a means of classifying the concentration of H+ ions and OH- ions in a solution.
The pH scale
- 7 is neutral (same number of H+ as OH-)
- lower numbers are acidic with more H+ than OH- ions
- higher numbers are basic with more OH- than H+